Using poor plastic to make a medical device can result in the failure of the product, regulatory nightmares, and even patient injuries. You may be concerned that your device will not pass stringent biocompatibility tests, to require expensive re-design and project delays at the expense of your business. The key to making a safe and confident decision is to know the main characteristics of medical-grade plastics. This is a guide that will take you through all that you should know to choose the right material.
The most effective method is to consider the biocompatibility, sterilizability, chemical resistance, and mechanical characteristics of the plastic in comparison to your device requirements and regulatory requirements, such as ISO 10993, to choose the most suitable biocompatible plastic. Important materials such as PEEK, medical grade Polycarbonate and Silicone have distinct advantages in various applications. The ultimate decision is always influenced by the duration of contact between the device and the body, the sterilization procedure needed and strength required. To make a decision, this guide disaggregates these factors to enable you make an informed decision.

Now that we’ve covered the basics, let’s dive deeper. Selecting the right material isn’t just about picking from a list; it’s about a systematic process of evaluation. I’ve seen many clients get overwhelmed by this, but it becomes much simpler when you know what to look for. It all starts with understanding the fundamental requirements that any plastic must meet to even be considered for medical use. So, what are those core non-negotiables you absolutely must verify?
What are the 4 material requirements for plastics used in medical devices?
Choosing the plastic may appear easy but in the case of medical machines, the risk is enormous. Mistaken decision is not merely a matter of production, but it is a matter of life and death. You must be concerned with the FDA or the CE tough audits and having your device become safe and reliable over the years. We can explain the four main pillars to which any medical grade plastic should be based. This will provide you with a good checklist of your selection of materials.
Medical device plastics need to meet four primary requirements: biocompatibility (it is safe to be in contact with the body), sterilizability (it can survive sterilization procedures, such as gamma, EtO, or autoclave), chemical resistance (it cannot be damaged by cleaning agents or body fluids), and mechanical properties (it must be strong enough, flexible enough and tough enough to be used in the intended application). These requirements can guarantee the safety, effectiveness, and reliability of the device in the entire lifecycle.

Let us discuss each of these requirements. Early in my life, a client presented himself to me with a design of a diagnostic tool housing. They had chosen a typical quality of ABS plastic since it was inexpensive and durable. The issue was that it was not medical grade. At the time we were talking about sterilization we knew that the gamma radiation they were to give me would make the plastic brittle and yellow. It also was not biocompatibility-tested. We were forced to change to a medical grade Polycarbonate which was more expensive in the short run but would save them both a product recall issue and a regulatory debacle in the future. This experience helped me to understand that all of these four points are not a list; they form the basis of a safe product.
1. Biocompatibility
This is the most important requirement. Biocompatibility means the material will not cause a negative reaction when it comes into contact with the human body. It won’t cause an allergic reaction, release toxic substances, or trigger an immune response. The international standard that governs this is ISO 10993. The level of testing required depends on how the device is used. A surgical tool handle that only touches a surgeon’s gloved hand has different requirements than a catheter that goes inside the body.
2. Sterilizability
Every medical device that isn’t single-use and pre-sterilized must be able to withstand sterilization to kill harmful microorganisms. However, not all plastics can handle all sterilization methods.
| Sterilization Method | How it Works | Suitable Plastics | Plastics to Avoid |
|---|---|---|---|
| Autoclave (Steam/Heat) | High-pressure steam at 121-134°C | PEEK, PPSU, Silicone, PP | Standard ABS, PVC, PC (can degrade) |
| Gamma Radiation | High-energy photons disrupt microbial DNA | PC, PE, Silicone, ABS | PP (can become brittle), Acetal (POM) |
| Ethylene Oxide (EtO) Gas | Low-temperature gas chemical sterilization | Most plastics, good for heat-sensitive materials | Materials that can absorb gas and are hard to vent |
| Electron Beam (E-beam) | High-energy electrons, similar to Gamma | PC, PE, ABS | PP, Acetal (POM) |
Choosing a plastic without confirming it can survive your chosen sterilization method is a recipe for disaster. The material could melt, crack, or change color, rendering the device useless.
3. Chemical Resistance
Medical environments are full of chemicals, from harsh cleaning agents and disinfectants to bodily fluids and drugs. The plastic you choose must be able to resist these substances without degrading, cracking, or swelling. For example, the housing of a blood glucose meter needs to withstand frequent wiping with alcohol-based cleaners. If the plastic cracks, the sensitive electronics inside could be damaged.
4. Mechanical Properties
Finally, the plastic must be able to do its job. This comes down to its physical and mechanical properties. Does it need to be rigid and strong like a surgical instrument handle? Or flexible and soft like medical tubing? Key properties to consider include tensile strength (resistance to being pulled apart), impact resistance (toughness), and flexural modulus (stiffness). You must match these properties to the real-world demands of the device.
What materials are biocompatible plastics?
Now you know what is needed but now you are presented with a long list of plastics, some with baffling acronyms like PEEK, PSU, and PEI. The problem of which of them suits you and fits your budget can be hectic. It is not desirable to over-engineer using an expensive material that you do not need and it is also not desirable to under-engineer and risk failure. We should put these materials into perspective and have a list of the most popular biocompatible plastics and their intended use.
A wide range of plastics might be biocompatible when manufactured as a specialty "medical grade." Typical ones are high-performance polymers such as PEEK, Radel(r) (PPSU) and Ultemtm (PEI), which are resilient and stable at high temperatures. Housings and components are made of engineering plastics such as medical grade Polycarbonate (PC) and ABS. Medical-grade Thermoplastic Elastomers (TPEs) and Silicone are good options in case of flexible applications. All of them provide varying ratios of properties, price, and regulatory compliance.
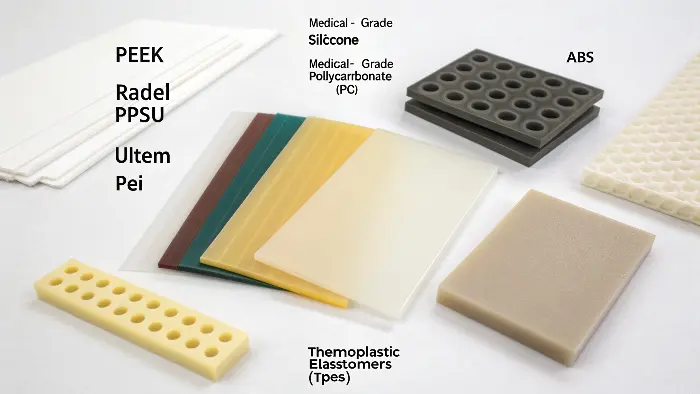
It’s important to remember that just because a material is called "Polycarbonate" doesn’t mean it’s biocompatible. You must source a specific medical grade that comes with documentation proving it has passed ISO 10993 testing. I always tell my clients to ask for the material’s regulatory data sheet. Let’s group these plastics to make them easier to understand.
High-Performance Plastics
These are the top-tier materials, offering exceptional performance but at a higher cost. They are often used for critical applications.
- PEEK (Polyetheretherketone): This is one of the strongest and most robust medical plastics. It has excellent mechanical properties, is resistant to almost all chemicals, and can withstand thousands of autoclave sterilization cycles. It’s so durable that it’s often used to replace metal in spinal implants and surgical instruments.
- Ultem™ (PEI): Ultem is known for its high strength, stiffness, and ability to handle high temperatures. It’s often amber and transparent, and like PEEK, it holds up very well to repeated sterilization, especially autoclaving. You’ll find it in surgical tool handles and trays.
- Radel® (PPSU): Polyphenylsulfone offers superior toughness and impact strength, even after being exposed to high temperatures and steam. It’s a great choice for devices that will be dropped or handled roughly and need to be sterilized many times.
Engineering Plastics
These materials offer a great balance of performance, processability, and cost. They are the workhorses for many non-implantable medical devices.
- Medical-Grade Polycarbonate (PC): PC is prized for its transparency, high impact strength, and rigidity. It’s perfect for anything that needs to be clear and tough, like IV connectors, dialysis filter housings, and surgical instrument handles.
- Medical-Grade ABS: ABS offers good stiffness and impact resistance at a lower cost than PC. It’s often used for device housings, covers, and casings where clarity is not needed.
- Medical-Grade Acetal (POM): Acetal is known for its high stiffness, low friction, and excellent wear resistance. This makes it ideal for moving parts in a device, like gears or clips in an insulin pen.
Flexible Plastics & Elastomers
When you need softness, flexibility, or a seal, these are the go-to materials.
- Medical-Grade Silicone: Silicone is extremely biocompatible and very stable over a wide range of temperatures. It’s soft, flexible, and used extensively for tubing, catheters, seals, gaskets, and even long-term implants.
- Medical-Grade TPEs (Thermoplastic Elastomers): TPEs behave like rubber but are processed like plastic. They are great for soft-touch grips on handles, flexible seals, and parts where you need both rigidity and flexibility in one component (overmolding).
What is the Biocompatibility Role in Material Selection?
What is the meaning of the term biocompatibility as it is applied to medical devices? It all comes down to ensuring that a substance can be used with the human tissues. The U.S Food and Drug Administration (FDA) has noted that biocompatibility by ISO 10993 standards of medical-grade plastics such as polypropylene or polycarbonate, is required. This is a vital necessity, with inadequacy on this matter resultant in severe complications. As an example, a recent article in the Journal of Biomedical Materials Research determined that non-biocompatible materials might cause inflammation, infection, and even systemic toxicity.
A report by the World Health Organization (WHO) of 2018 indicates that the use of medical devices is up to 10 percent of the global population, which means that the safety of medical devices is a global issue. There is no doubt that the biocompatibility of the used materials is of paramount importance whether in the case of medical products such as a syringe or in the situation of a complex implant. Since such devices are usually in direct contact with the body over long periods of time, as pointed out in 2017 by a study published in the Journal of Clinical Medicine, some of the implants can stay inside the body over ten years, it is important to make sure that they are safe.
These medical-grade plastics are subjected to a sequence of stringent tests in order to be safe when they are in contact with people. The tests discussed in the American Society for Testing and Materials (ASTM) are cytotoxicity, sensitization, and irritation or intracutaneous reactivity tests among others, so that negative reactions such as inflammation or toxicity can be ruled out. However, it is not only the plastic that needs to undergo biocompatibility tests, but the entire production process, along with any additives or coating, should do the same. In a report published by the Regulatory Affairs Professionals Society (RAPS) in 2020, despite the changes in manufacturing processes being minor, they may impact the biocompatibility of a material.
The importance of biocompatibility is hard to overestimate. It is the key to patient safety in the medical equipment industry.
What Plastic is most Commonly used in Medical Devices?
You have already witnessed the alternatives and now you are most likely asking what the most frequent materials of most companies are. You would like to take a safe, tried and tested decision without having to reinvent the wheel and have a secure supply chain and already tested manufacturing procedures. Now we can discuss the workhorses of the medical plastics industry- the materials you will find in use time and again, and why they have become so popular.
Although the optimal plastic is dependent on the use, Polypropylene (PP) and Polycarbonate (PC) are some of the most common materials used in medical equipment. PP is very popular in single-use items such as syringes, containers and packaging because of low cost, chemical resistance and autoclave sterilization. PC is also preferred due to its transparency, high impact strength and rigidity which makes it perfect in houses, connectors and surgical instruments which require durability and visibility.

When I walk through a hospital, I see these two plastics everywhere. They cover two very different but massive areas of the medical device market: disposables and reusables. Their popularity isn’t an accident; it’s because they provide a fantastic, reliable solution for a huge range of common problems. But they aren’t the only major players. Let’s look at why these materials are so dominant.
Why Polypropylene (PP) is a Leader
Think of all the single-use items in healthcare: syringes, disposable beakers, suture packaging, and sample containers. The vast majority are made from medical-grade PP. The reason is simple: it offers the perfect combination of properties for high-volume, disposable products.
- Low Cost: When you’re making millions of an item that will be used once and thrown away, cost is a primary driver. PP is one of the most affordable plastics available.
- Good Chemical Resistance: It holds up well against many common alcohols and solvents.
- Autoclave Resistant: This is a huge advantage. It can be sterilized with high-pressure steam, one of the most common and reliable sterilization methods.
- Living Hinge Capability: PP can be molded with a "living hinge," a thin section of plastic that can be flexed thousands of times without breaking. This is perfect for container lids.
Why Polycarbonate (PC) is a Workhorse
For devices that need to be durable, reusable, and often transparent, medical-grade PC is a top choice. It’s a step up in performance and price from PP, and it delivers in critical areas.
- Clarity: PC is naturally transparent, which is essential for devices where you need to monitor fluid flow, like IV components or blood filters.
- Impact Strength: PC is incredibly tough and shatter-resistant. This is vital for device housings or instruments that might be dropped. I remember a client developing a handheld scanner; we chose PC for the housing specifically so it could survive a 4-foot drop onto concrete, a common hospital accident.
- Rigidity and Dimensional Stability: It holds its shape well under stress and at moderate temperatures, ensuring that parts fit together precisely. This is key for complex assemblies and connectors.
The Unsung Hero: Polyethylene (PE)
Polyethylene, especially Ultra-High-Molecular-Weight Polyethylene (UHMWPE), is another hero material, but for a very specific and critical application: orthopedic implants. UHMWPE has incredibly low friction and outstanding wear resistance. For decades, it has been the gold standard material for the "socket" part of hip and knee joint replacements, rubbing against a metal or ceramic ball. Its durability has allowed millions of people to walk pain-free.
What are the Primary Benefits of Using Biocompatible Plastics Over Metals in Medical Devices?
Some main benefits are as discussed below:
1. Lightweight Flexible Structure
The most apparent advantage of biocompatible plastics over metals in medical devices is that they are flexible and lightweight. Plastics play a huge role in minimizing the weight of appliances such as prosthetics, catheters, and implants, improving patient mobility and comfort. The flexibility also allows manufacturers to develop complex and ergonomic shapes that are difficult to mimic in metals. This results in simpler-to-use medical devices, which are less invasive for the patient and more adaptable to evolving anatomical requirements.
2. Excellent Biocompatibility
Biocompatible plastics are specifically engineered to react safely with human tissues and body fluids. PEEK (polyether ether ketone), PTFE (polytetrafluoroethylene), PMMA (polymethyl methacrylate), and medical-grade polypropylene are some of the materials that show high tolerance in the body. Unlike some metals, which can trigger immune reactions or inflammation, these polymers minimize the chances of adverse reactions. This makes them ideal for long-term implants, surgery tools, and devices that remain in prolonged contact with tissue or blood.
3. Better Sterilizability
Yet another significant benefit of biocompatible plastics is that they can withstand repeated sterilization processes without compromising their properties. Most medical-grade plastics can survive gamma radiation, ethylene oxide gas sterilization, and even steam autoclaving. This enables manufacturers to ensure good hygiene practices while ensuring the devices remain resistant to repeated clinical use without compromising safety. Such reliability makes them essential for both single-use and reusable instruments.
4. Customized Mechanical Characteristics
The flexibility to modify plastics’ mechanical characteristics to suit certain application requirements is a significant additional advantage. It is possible to engineer plastics for increased strength, flexibility, or impact resistance by adding additives, fillers, or blending.
This degree of personalization enables designers to produce materials that nearly resemble the mechanical performance needed for various medical devices, from soft tubing to hard implants, something that is difficult to accomplish with metals.
5. Decreased Allergic Responses
Patients may occasionally experience adverse reactions or hypersensitivity to metals like nickel, chromium, or cobalt. Inflammation, discomfort, or even implant rejection may result from this. Because they are hypoallergenic and non-reactive, biocompatible plastics minimize this worry.
6. Cost Efficiency
From the production perspective, biocompatible plastics are considerably cheaper to produce and manufacture compared to metals. Techniques such as injection molding, extrusion, and thermoforming allow for high-volume, quick manufacturing in large volumes at reduced cost. Plastics are therefore ideal for disposable or one-time use medical devices, where cost reduction is essential without diminishing quality or performance. Reduced costs of production also mean reduced healthcare costs.
7. Radiolucency (X-ray Transparency)
Plastics are radiolucent in nature, i.e., they don’t interfere with imaging equipment such as X-rays, CT scans, or MRIs. Metals, by contrast, cast shadows or distortions in medical images that make it difficult for physicians to see inside tissues or where the implant is located. The use of radiolucent plastics enables physicians to monitor patients more accurately, establish the status of healing, and carry out diagnostics without the necessity of removing the device.
Finally, biocompatible plastics also provide an array of performance, safety and design flexibility which in many cases, cannot be matched by metals. Their benefits making them a material of choice in most of the modern medical devices include their lightweight structure, chemical stability, high biocompatibility and cost effectiveness.
Going forward as medical sector keeps changing its paradigm, adoption of the high-performance plastics will be instrumental in realizing safer, more effective, and sustainable healthcare products.
What is the best biocompatible plastic?
Everybody wants to have the best material to make their product out of. It is a natural and significant question to put. However, you may fear that choosing the most expensive or highest-performing plastic, such as PEEK, would be spending unnecessary money because there is no value addition to your particular device. Conversely, you are afraid that a good enough material could contain a latent fault that will manifest itself in the future. The fact is that there is no such thing as the best plastic. The correct plastic, however, most certainly does. Let’s explore how to find it.
No better biocompatible plastic exists, the right solution is all about the application and the need. PEEK may be preferred because of its strength when a surgical instrument needs reuse and requires harsh sterilization on numerous occasions. Polycarbonate is one of the best options when it comes to having a clear and strong housing on a diagnostic machine. In a long-term implant that is flexible, then medical-grade Silicone is usually the best. The most appropriate material is the one that fulfills all your device functionality, regulation and budget requirements without being over-engineered.

I guide my clients through this decision process every day. The answer is always found by asking the right questions about the device itself. A business owner like you, Michael, needs a material that is not just technically sound but also commercially viable. Paying for the performance of PEEK when medical-grade PP would do the job perfectly is not a good business decision. Let’s break down the thought process for finding the right plastic.
Factor 1: How Will It Contact the Body?
This is the first question to ask, as it dictates the level of biocompatibility testing required under ISO 10993.
- Surface Contact: For devices that only touch intact skin, like a monitor housing or an equipment cart, the requirements are less stringent. Materials like medical-grade ABS or PC are often sufficient.
- Short-Term Contact: For devices that contact the body for less than 24 hours, like a catheter or a drug delivery system, the material needs a higher level of biocompatibility. Medical-grade PC, Silicone, and TPEs are common here.
- Long-Term Implants: For anything staying in the body for more than 30 days, like a spinal cage or a joint replacement, only the most rigorously tested materials can be used. This is where you see implant-grade PEEK, Silicone, and UHMWPE.
Factor 2: How Will It Be Sterilized?
As we discussed, this is a critical gate. You must match the material to the sterilization method. If your company’s standard procedure is gamma radiation, you should avoid using Polypropylene. If you require repeated autoclaving, PEEK or PPSU are far better choices than PC. Always create a shortlist of materials that can reliably withstand your intended sterilization cycle.
Factor 3: What Are the Physical and Mechanical Demands?
Think about the device’s daily life.
- Rigid or Flexible? A surgical handle needs to be stiff (high flexural modulus), while tubing needs to be flexible (low flexural modulus).
- Clear or Opaque? If you need to see through it, your choices narrow to PC, clear TPEs, or other transparent polymers.
- Toughness: Will the device be dropped or subject to impact? If so, prioritize materials with high impact strength like PC or PPSU.
- Chemical Exposure: Will it be wiped down daily with harsh disinfectants? If so, check the chemical resistance charts for materials like PEEK or PP, which generally perform better than PC or ABS against aggressive chemicals.
Factor 4: What is the Cost vs. Performance Trade-off?
This is where business sense comes in. Don’t pay for performance you don’t need. PEEK can cost over 50 times more than PP per pound. If your application is a single-use container, using PEEK would be absurd. The goal is to find the lowest-cost material that meets all of your technical, safety, and regulatory requirements. This ensures your product is not only safe and effective but also competitive in the market.
Conclusion
The selection of plastic to use when making a medical device is a very critical process that weighs between safety, functionality and cost. It is not about identifying the one and only best material, but a systematic approach of aligning the properties of a material to the unique requirements of your device. With the four basic needs of biocompatibility, sterilizability, chemical resistance and mechanical strength, you can create a pillar to a safe and reliable product that fulfills all regulatory requirements and benefits patients.
